B07: Peritoneal Immune States Link Gut and Liver Pathology in Cirrhosis
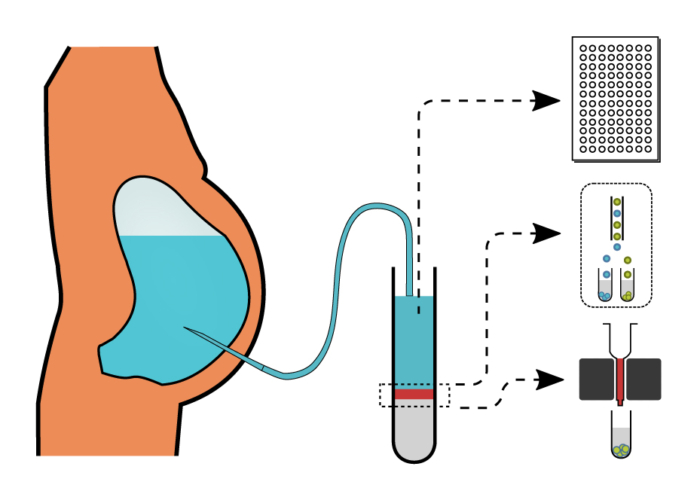
Spontaneous bacterial infections are the result of gut bacterial translocation, precipitate acute-on-chronic liver failure, and cause significant mortality in patients with end-stage liver disease. In addition to gut dysbiosis and disturbed intestinal permeability, mounting evidence suggests a failure of immunological control of gut-derived inflammation and infection in patients with cirrhosis. We previously showed that inflammation and infection critically shape human peritoneal macrophage composition and phenotype and indicate complications of cirrhosis.
We therefore hypothesize that the peritoneal immune compartment provides an important link between gut and liver pathology with distinct disease-associated peritoneal immune states, which contribute to systemic inflammation and control gut-derived spontaneous infections in patients with decompensated cirrhosis and ascites. The main purpose of the study is to characterize such disease- associated peritoneal immune states and to investigate strategies to modulate activation, differentiation and migration of distinct peritoneal immune cell subsets in cirrhosis.
In order to systematically assess peritoneal immunity in decompensated cirrhosis, we aim
- To characterize the composition and function of the human peritoneal immune cells, during and after disease-modifying events,
- To investigate the epigenetic landscape of transcriptionally active and closed DNA sites associated with altered immune memory in human peritoneal macrophages, validating possible therapeutic targets to modulate peritoneal inflammation, and
- To link peritoneal T cell activation and exhaustion to gut permeability and bacterial translocation in cirrhosis.
Manipulating peritoneal immunity in patients with decompensated cirrhosis may represent a novel strategy targeting the gut-liver axis in order to prevent bacterial infections, dampen inflammation, and improve survival.
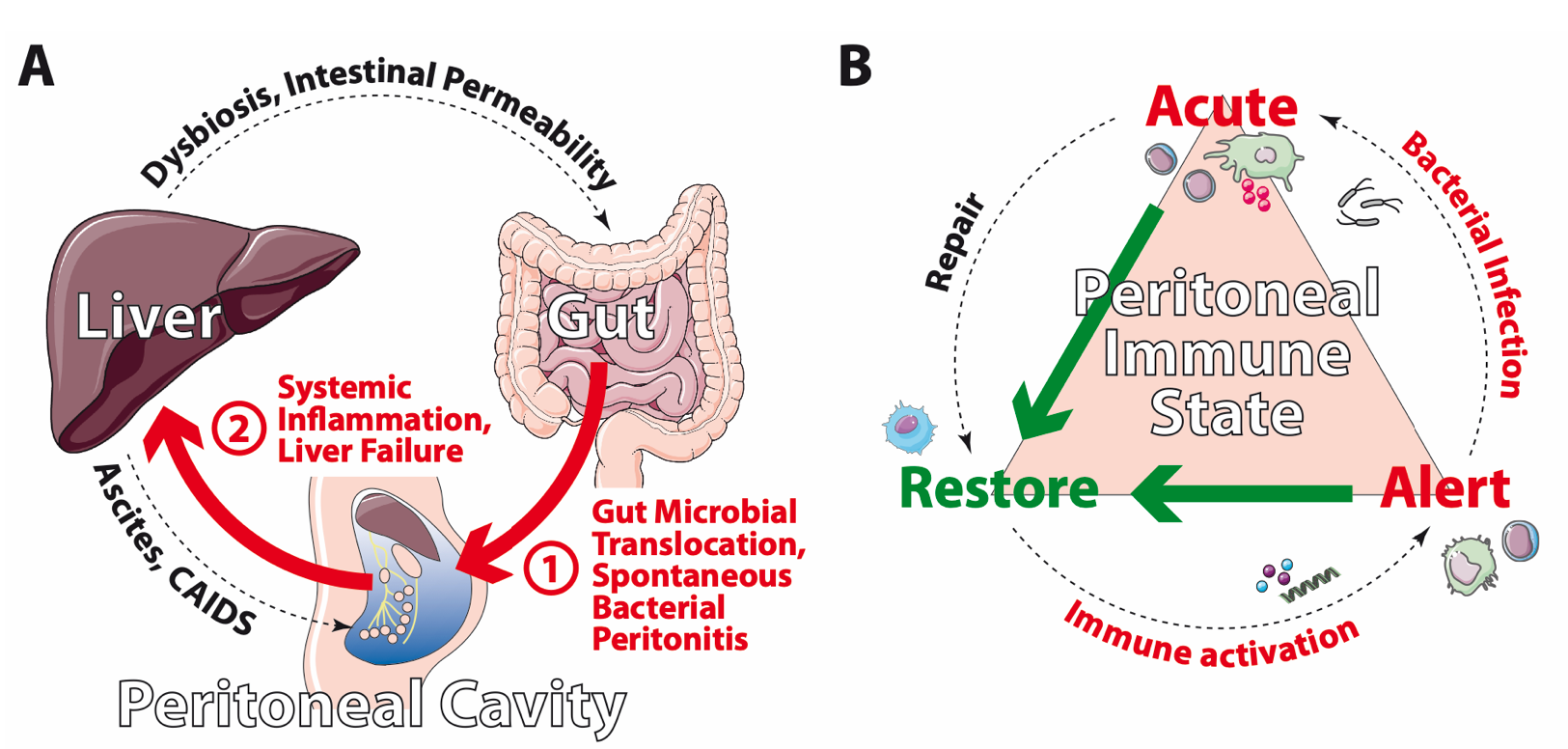
Schematic representation (A) of the functional circuit of gut, liver, and peritoneum and (B) proposed peritoneal immune states: the “alert state” in presence of bacterial products, danger-associated molecular patterns (DAMPs) and primed/activated immune cells, the “acute state” in spontaneous bacterial peritonitis (SBP), and the anti-inflammatory, restorative state. CAIDS: Cirrhosis-associated Immune Dysfunction Syndrome.